
Chemistry, 27.08.2019 17:10 lrich20200
Amethod used by the u. s. environmental protection agency (epa) for determining the concentration of ozone in air is to pass the sample through a "bubbler" containing sodium iodide, which removes the ozone according to the chemical equation: o3(g) + 2 nai(aq) + h2o(l) → o2(g) + i2(s) + 2 naoh(aq) how many moles of sodium iodide are needed to remove 4.54 ✕ 10−6 mol of o3? mol nai how many milligrams of sodium iodide are needed to remove 13.31 mg of o3?

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:02
When undergoing chemical reactions where does the reactant combine to the enzyme?
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

You know the right answer?
Amethod used by the u. s. environmental protection agency (epa) for determining the concentration of...
Questions

Mathematics, 05.03.2021 16:10


English, 05.03.2021 16:10



Physics, 05.03.2021 16:10


Mathematics, 05.03.2021 16:10




Advanced Placement (AP), 05.03.2021 16:10

Social Studies, 05.03.2021 16:10



English, 05.03.2021 16:10


Mathematics, 05.03.2021 16:10

Mathematics, 05.03.2021 16:10

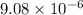 moles
moles 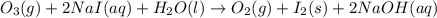
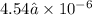 moles of ozone is removed by =
moles of ozone is removed by =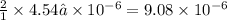 moles of sodium iodide.
moles of sodium iodide.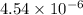 moles of
moles of 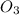
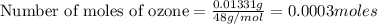
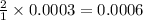 moles of sodium iodide.
moles of sodium iodide.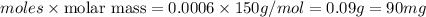 (1g=1000mg)
(1g=1000mg)

