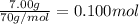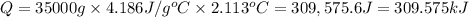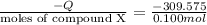Chemistry, 27.08.2019 17:10 sakurauchiha913
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter containing 35.00kg of water at 25°c. the temperature of the water is observed to rise by 2.113°c. (you may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) calculate the standard heat of formation of compound x at 25°c. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. [could you show a step by step way to solve this]

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter conta...
Questions


Mathematics, 23.04.2021 09:40

Arts, 23.04.2021 09:40

Mathematics, 23.04.2021 09:40


Mathematics, 23.04.2021 09:40

History, 23.04.2021 09:40

Mathematics, 23.04.2021 09:40


Chemistry, 23.04.2021 09:40



Computers and Technology, 23.04.2021 09:40

Spanish, 23.04.2021 09:40


Chemistry, 23.04.2021 09:40


Mathematics, 23.04.2021 09:40


Mathematics, 23.04.2021 09:40