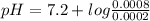Chemistry, 27.08.2019 17:10 desikayla2013
Ph indicator. a dye that is an acid and appears as different colors in its protonated and deprotonated forms can be used as a ph indicator. suppose that you have a 0.001 m solution of a dye with a p k a of 7.2. from the color, the concentration of the protonated form is found to be 0.0002 m. assume that the remainder of the dye is in the deprotonated form. what is the ph of the solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
Ph indicator. a dye that is an acid and appears as different colors in its protonated and deprotonat...
Questions




Mathematics, 25.07.2019 05:00


History, 25.07.2019 05:00





Biology, 25.07.2019 05:00






Biology, 25.07.2019 05:00


Biology, 25.07.2019 05:00

![pH = p_{ka} +log\frac{[Salt]}{[Acid]}](/tpl/images/0202/9224/babd2.png)