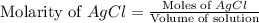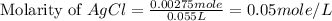Suppose 0.701g of iron(ii) chloride is dissolved in 50.ml of a 55.0mm aqueous solution of silver nitrate.
calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn't change when the iron(ii) chloride is dissolved in it.
be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Clouds form when water vapor to form small droplets. a. humidifies b. condenses c. evaporates d. precipitates
Answers: 2


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Suppose 0.701g of iron(ii) chloride is dissolved in 50.ml of a 55.0mm aqueous solution of silver nit...
Questions








Biology, 21.08.2019 23:00




Social Studies, 21.08.2019 23:00


Physics, 21.08.2019 23:00

English, 21.08.2019 23:00

English, 21.08.2019 23:00

Mathematics, 21.08.2019 23:00


History, 21.08.2019 23:00

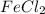 = 0.701 g
= 0.701 g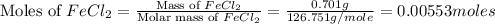
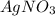 .
.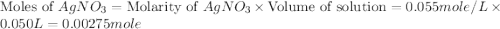
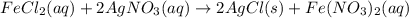
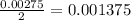 moles of
moles of 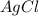 .
.