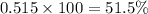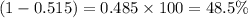The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x, one weighing 59 g/mol, and the other weighing 61 g/mol. what is the relative abundance of each?
possible answers:
50% x-59, 50% x-61
75% x-59, 25% x-61
63% x-59, 37% x-61
51.5% x-59, 48.5% x-61
48.5% x-59, 51.5% x-61

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x...
Questions



Mathematics, 24.05.2021 19:40


Mathematics, 24.05.2021 19:40

Mathematics, 24.05.2021 19:40

Chemistry, 24.05.2021 19:40



Chemistry, 24.05.2021 19:40


Mathematics, 24.05.2021 19:40

History, 24.05.2021 19:40

Mathematics, 24.05.2021 19:40

Biology, 24.05.2021 19:40

Mathematics, 24.05.2021 19:40



Social Studies, 24.05.2021 19:40

Mathematics, 24.05.2021 19:40

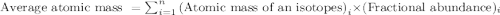 .....(1)
.....(1)![59.97 g/mol=[(59 g/mol\times x)+(61 g/mol\times (1-x))]\\\\x=0.515](/tpl/images/0202/9296/4c349.png)