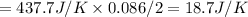Chemistry, 27.08.2019 17:20 siriuskitwilson9408
What is the change in entropy when 7.61 ml of liquid benzene (c6h6, d = 0.879 g/ml) is combusted in the presence of 22.3 l of oxygen gas, measured at 298 k and 1 atm pressure? (r = 0.0821 l · atm/(k · mol)) 2c6h6(l) + 15o2(g) → 12co2(g) + 6h2o(l); δs° = –437.7 j/k at 298 k a. 436 j/k b. 37.4 j/k c. 398 j/k d. 45.3 j/k e. 18.7 j/k

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
What is the change in entropy when 7.61 ml of liquid benzene (c6h6, d = 0.879 g/ml) is combusted in...
Questions

History, 05.10.2020 03:01


History, 05.10.2020 03:01

Mathematics, 05.10.2020 03:01

English, 05.10.2020 03:01


History, 05.10.2020 03:01


Mathematics, 05.10.2020 03:01


Social Studies, 05.10.2020 04:01

Mathematics, 05.10.2020 04:01

History, 05.10.2020 04:01

History, 05.10.2020 04:01

Mathematics, 05.10.2020 04:01


Mathematics, 05.10.2020 04:01

Physics, 05.10.2020 04:01

Physics, 05.10.2020 04:01

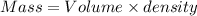
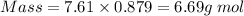
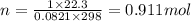
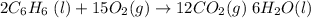
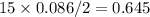 mol of O2
mol of O2