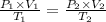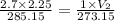Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
Asample of neon is collected at 2.7 atm and 12.0 oc. it has a volume of 2.25 l. what would be the vo...
Questions

Mathematics, 16.06.2020 21:57




Mathematics, 16.06.2020 21:57


Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57

Social Studies, 16.06.2020 21:57

History, 16.06.2020 21:57




Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57