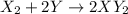Chemistry, 27.08.2019 18:30 hcpscyruscm
Ascientist finds that one volume of an unknown gas x combines with two volumes of another gas y to form two volumes of a new gas with a formula xy2. are any of the gases diatomic?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
Ascientist finds that one volume of an unknown gas x combines with two volumes of another gas y to f...
Questions

History, 19.10.2020 01:01


Mathematics, 19.10.2020 01:01



English, 19.10.2020 01:01


Social Studies, 19.10.2020 01:01


Mathematics, 19.10.2020 01:01



Mathematics, 19.10.2020 01:01

English, 19.10.2020 01:01

Business, 19.10.2020 01:01

Health, 19.10.2020 01:01


Chemistry, 19.10.2020 01:01


Mathematics, 19.10.2020 01:01

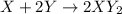
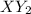 cannot be diatomic as the formula contains 3 atoms.
cannot be diatomic as the formula contains 3 atoms.