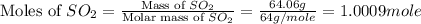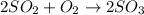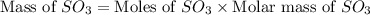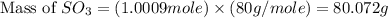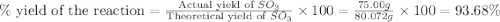In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulfur trioxide. using the equation, 2so2 (g) + o2 imported asset 2so3 (g), if 64.06g of sulfur dioxide is given an opportunity to react with an excess of oxygen to produce 75.00 g of sulfur trioxide, what is the percent yield of this reaction? 46.83% 60.25% 75.55% 93.68%

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1



Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3
You know the right answer?
In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulf...
Questions



Chemistry, 20.04.2020 23:11

Chemistry, 20.04.2020 23:11




Mathematics, 20.04.2020 23:11




History, 20.04.2020 23:11

Mathematics, 20.04.2020 23:11

History, 20.04.2020 23:11

Mathematics, 20.04.2020 23:11





English, 20.04.2020 23:12

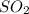 = 64.06 g
= 64.06 g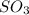 = 80 g/mole
= 80 g/mole