

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

You know the right answer?
Boron sulfide, b2s3(s), reacts violently with water to form dissolved boric acid (h3bo3) and hydroge...
Questions



Mathematics, 25.10.2020 07:50

English, 25.10.2020 07:50

Social Studies, 25.10.2020 07:50

Mathematics, 25.10.2020 07:50

History, 25.10.2020 07:50



Mathematics, 25.10.2020 07:50

Advanced Placement (AP), 25.10.2020 07:50

Mathematics, 25.10.2020 07:50


Mathematics, 25.10.2020 07:50


Physics, 25.10.2020 07:50


Mathematics, 25.10.2020 07:50

Mathematics, 25.10.2020 07:50

 + H₂O
+ H₂O → H₃BO₃
→ H₃BO₃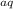 + H₂S
+ H₂S


