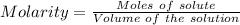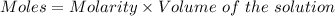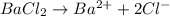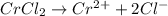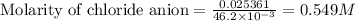Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
In the laboratory a student combines 28.7 ml of a 0.240 m barium chloride solution with 17.5 ml of a...
Questions

Geography, 17.09.2019 13:50

Health, 17.09.2019 13:50


Mathematics, 17.09.2019 13:50

Mathematics, 17.09.2019 13:50

Mathematics, 17.09.2019 13:50


English, 17.09.2019 13:50


Biology, 17.09.2019 13:50


Mathematics, 17.09.2019 13:50


Mathematics, 17.09.2019 13:50


Chemistry, 17.09.2019 13:50

Mathematics, 17.09.2019 13:50

Mathematics, 17.09.2019 13:50