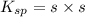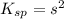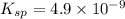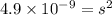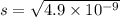Chemistry, 28.08.2019 02:30 blackbetty79
What is the concentration of ca2+(aq) in a saturated solution of caco3? (note: the solubility product constant ksp for caco3 is 4.9 × 10–9.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1
You know the right answer?
What is the concentration of ca2+(aq) in a saturated solution of caco3? (note: the solubility prod...
Questions


Mathematics, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40





Mathematics, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40




Mathematics, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

![K_{sp}=\left [ Ca^{2+} \right ]\left [ CO_3^2- \right ]](/tpl/images/0204/3244/19e89.png)