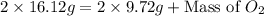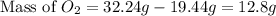Chemistry, 28.08.2019 17:20 chloesmolinski0909
The law of conservation of mass states that mass is neither created nor destroyed during a chemical reaction. this can be gleaned from the third postulate in dalton's series. magnesium oxide decomposes into magnesium and oxygen. if 16.12 g of magnesium oxide decomposes to form 9.72 g of magnesium, what mass of oxygen gas is also released in the reaction?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
The law of conservation of mass states that mass is neither created nor destroyed during a chemical...
Questions

Mathematics, 16.10.2019 02:30


Business, 16.10.2019 02:30

Mathematics, 16.10.2019 02:30

Mathematics, 16.10.2019 02:30




Computers and Technology, 16.10.2019 02:30



Mathematics, 16.10.2019 02:30

History, 16.10.2019 02:30


Health, 16.10.2019 02:30

Health, 16.10.2019 02:30

Mathematics, 16.10.2019 02:30



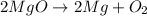
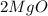 = Total mass of
= Total mass of 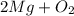
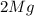 + Mass of
+ Mass of 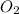
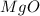 = 16.12 grams
= 16.12 grams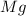 = 9.72 grams
= 9.72 grams