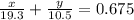Consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3 . the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, respectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains, calculate the percentage of gold (by mass) in the jewelry.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3 . the jewelry cont...
Questions

Mathematics, 15.01.2022 15:00


Mathematics, 15.01.2022 15:00


Mathematics, 15.01.2022 15:00


English, 15.01.2022 15:10

Chemistry, 15.01.2022 15:10


Mathematics, 15.01.2022 15:10

Geography, 15.01.2022 15:10


Mathematics, 15.01.2022 15:10

Mathematics, 15.01.2022 15:10

Mathematics, 15.01.2022 15:10

Physics, 15.01.2022 15:10

Mathematics, 15.01.2022 15:10



Mathematics, 15.01.2022 15:10