
Determine the number of moles of the compound and determine the number of moles of each type of atom in each of the following. a. 25.0 g of propylene, c3h6b. 3.06 x 10^-3g of the amino acid glycine, c2h5no2c. 25 lb of the herbicide treflan, c13h16n2o4f (1lb = 454 g)d. 0.125 kg of the insecticide paris green, cu4(aso3)2(ch3co2)2e. 325 mg of aspirin, c6h4(co2h)(co2ch3)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Determine the number of moles of the compound and determine the number of moles of each type of atom...
Questions



Mathematics, 22.12.2020 03:50

SAT, 22.12.2020 03:50


Chemistry, 22.12.2020 03:50

English, 22.12.2020 03:50

Business, 22.12.2020 03:50

Mathematics, 22.12.2020 03:50

Mathematics, 22.12.2020 03:50

History, 22.12.2020 03:50

Mathematics, 22.12.2020 03:50


Mathematics, 22.12.2020 03:50

Mathematics, 22.12.2020 03:50




Mathematics, 22.12.2020 04:00

English, 22.12.2020 04:00

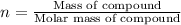
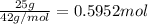
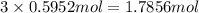 of carbon
of carbon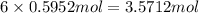 of hydrogen
of hydrogen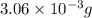
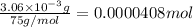
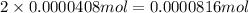 of carbon atom
of carbon atom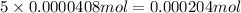 of hydrogen atom
of hydrogen atom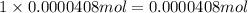 of nitrogen
of nitrogen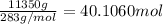
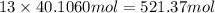 of carbon atom
of carbon atom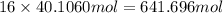 of hydrogen atom
of hydrogen atom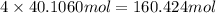 of oxygen atom
of oxygen atom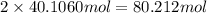 of nitrogen atom
of nitrogen atom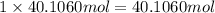 of fluorine
of fluorine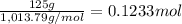
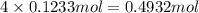 of carbon atom
of carbon atom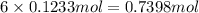 of hydrogen atom
of hydrogen atom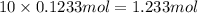 of oxygen atom
of oxygen atom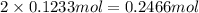 of fluorine
of fluorine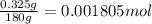
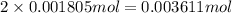 of carbon atom
of carbon atom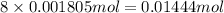 of hydrogen atom
of hydrogen atom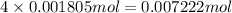 of oxygen atom
of oxygen atom

