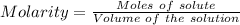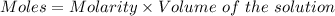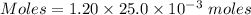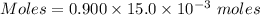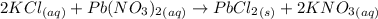Chemistry, 28.08.2019 17:30 leomessifanboy678
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii) nitrate solution and this precipitation reaction occurs: 2 kcl(aq)+pb(no3)2(aq)→pbcl2(s)+2 kno3(aq) the solid pbcl2 is collected, dried, and found to have a mass of 2.45 g. determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

You know the right answer?
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii)...
Questions

Arts, 17.12.2020 18:50

Chemistry, 17.12.2020 18:50



Mathematics, 17.12.2020 18:50

Chemistry, 17.12.2020 18:50

Social Studies, 17.12.2020 18:50



Biology, 17.12.2020 18:50

Physics, 17.12.2020 18:50



English, 17.12.2020 18:50

Biology, 17.12.2020 18:50

Mathematics, 17.12.2020 18:50

Mathematics, 17.12.2020 18:50


Mathematics, 17.12.2020 18:50

Mathematics, 17.12.2020 18:50