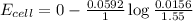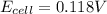Chemistry, 28.08.2019 17:30 dawsoncollins4117
Avoltaic cell is constructed with two silver-silver chloride electrodes, where the half-reaction isagcl (s) + e- ? ag (s) + cl-(aq) e° = +0.222 vthe concentrations of chloride ion in the two compartments are 0.0156 m and 1.55 m, respectively. the cell emf is v. a 0.232b 22.1c 0.212d 0.00223e 0.118

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Avoltaic cell is constructed with two silver-silver chloride electrodes, where the half-reaction isa...
Questions


Chemistry, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20



History, 06.05.2021 22:20


Mathematics, 06.05.2021 22:20



Mathematics, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20

French, 06.05.2021 22:20




History, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20

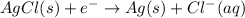

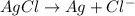
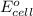 is equal to zero.
is equal to zero.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Cl^-{cathode}}{[Cl^-{anode}]}](/tpl/images/0206/1459/abfb9.png)
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Cl^-{diluted}}{[Cl^-{concentrated}]}](/tpl/images/0206/1459/6da9b.png)
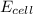 = ?
= ?![[Cl^-{diluted}]](/tpl/images/0206/1459/790d6.png) = 0.0156 M
= 0.0156 M![[Cl^-{concentrated}]](/tpl/images/0206/1459/56eb0.png) = 1.55 M
= 1.55 M