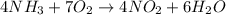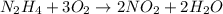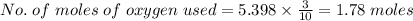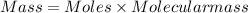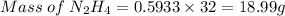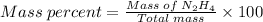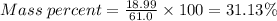Agas contains a mixture of nh3(g) and n2h4(g), both of which react with o2(g) to form no2(g) and h2o(g). the gaseous mixture (with an initial mass of 61.00 g) is reacted with 10.00 moles o2, and after the reaction is complete, 4.062 moles o2 remains. calculate themass percent of n2h4(g) in the original gaseous mixture.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
Agas contains a mixture of nh3(g) and n2h4(g), both of which react with o2(g) to form no2(g) and h2o...
Questions

Mathematics, 11.03.2021 04:00

Health, 11.03.2021 04:00


Mathematics, 11.03.2021 04:00

Mathematics, 11.03.2021 04:00

Mathematics, 11.03.2021 04:00




Mathematics, 11.03.2021 04:00



Arts, 11.03.2021 04:00


Mathematics, 11.03.2021 04:10


Physics, 11.03.2021 04:10