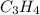Chemistry, 28.08.2019 21:10 heavenwagner
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone and phenol in the chemical industry. combustion of 47.6 mg cumene produces some co2 and 42.8 mg water. the molar mass of cumene is between 115 and 125 g/mol. determine the empirical and molecular formulas.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone a...
Questions

Biology, 12.01.2022 18:50

Geography, 12.01.2022 18:50


English, 12.01.2022 18:50




English, 12.01.2022 18:50


English, 12.01.2022 18:50

Social Studies, 12.01.2022 18:50




English, 12.01.2022 19:00





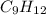
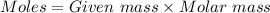
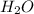 = 42.8/18 = 2.3778×10⁻³ moles
= 42.8/18 = 2.3778×10⁻³ moles