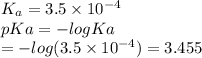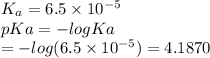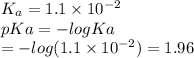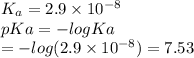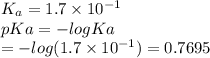Chemistry, 28.08.2019 21:30 Andrewecolt1993
Which of the following acids (listed with ka values) and their conjugate base would form a buffer with a ph of 2.34? a. hf, ka = 3.5 x 10-4 b. c6h5cooh, ka = 6.5 x 10-5 c. hclo2, ka = 1.1 x 10-2 d. hclo, ka = 2.9 x 10-8 e. hio3, ka = 1.7 x 10-1

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1


Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
Which of the following acids (listed with ka values) and their conjugate base would form a buffer wi...
Questions


English, 23.10.2021 17:10


Spanish, 23.10.2021 17:10

Mathematics, 23.10.2021 17:10

Mathematics, 23.10.2021 17:10





Health, 23.10.2021 17:10

Chemistry, 23.10.2021 17:10

Mathematics, 23.10.2021 17:10





Chemistry, 23.10.2021 17:10

Mathematics, 23.10.2021 17:10