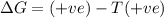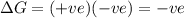For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one would predict thata. δh˚ is negative and δs˚ is negativeb. δh˚ is positive and b. δs˚ is negativec. δh˚ is positive and δs˚ is positived. δh˚ is negative and δs˚ is positive

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one wou...
Questions

Arts, 15.12.2020 21:10

Biology, 15.12.2020 21:10



Mathematics, 15.12.2020 21:10

English, 15.12.2020 21:10

Biology, 15.12.2020 21:10

Biology, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10


Mathematics, 15.12.2020 21:10



Biology, 15.12.2020 21:10

Mathematics, 15.12.2020 21:10

World Languages, 15.12.2020 21:10

Chemistry, 15.12.2020 21:10

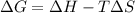
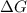 = Gibbs free energy
= Gibbs free energy 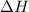 = enthalpy change
= enthalpy change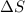 = entropy change
= entropy change 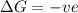
 >
> 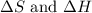 both have positive values.
both have positive values.