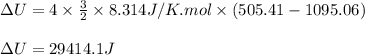Chemistry, 28.08.2019 23:00 michaellangley
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas (cv = 3/2 r). the compression reduces the volume of the gas from 0.26 m^3 to 0.12 m^3. the change in the internal energy of the gas, in kj is ("^3" means to the power of 3)

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas...
Questions




Biology, 22.08.2019 19:00

Biology, 22.08.2019 19:00

Mathematics, 22.08.2019 19:00




World Languages, 22.08.2019 19:00

Health, 22.08.2019 19:00



Mathematics, 22.08.2019 19:00

Computers and Technology, 22.08.2019 19:00



Mathematics, 22.08.2019 19:00

Mathematics, 22.08.2019 19:00

Chemistry, 22.08.2019 19:00

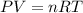
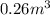
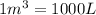
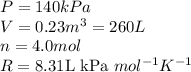
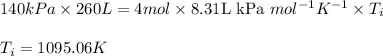
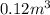
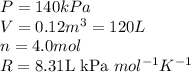
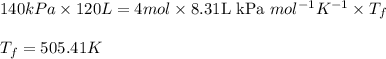
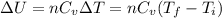
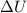 = change in internal energy = ?
= change in internal energy = ?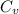 = heat capacity at constant volume =
= heat capacity at constant volume = 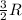
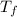 = final temperature = 1095.06 K
= final temperature = 1095.06 K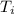 = initial temperature = 505.41 K
= initial temperature = 505.41 K