
Chemistry, 29.08.2019 01:20 emmapizano
Use the following, balanced equation for the question below: 2al(s) + 3cucl2(aq) → 3cu(s) + 2alcl3(aq) how many moles of copper metal will be produced from 10.0 moles of aluminum metal? 10.0 moles of copper 15.0 moles of copper 20.0 moles of copper 30.0 moles of copper

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Use the following, balanced equation for the question below: 2al(s) + 3cucl2(aq) → 3cu(s) + 2alcl3(...
Questions

English, 20.11.2021 14:00


Computers and Technology, 20.11.2021 14:00


History, 20.11.2021 14:00





Mathematics, 20.11.2021 14:00








Mathematics, 20.11.2021 14:00


Mathematics, 20.11.2021 14:00

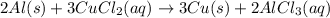
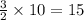 moles of copper.
moles of copper.

