
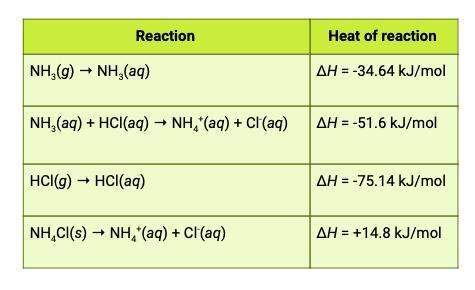
The heat of reaction for the process described in (a) can be determined by applying hess's law. the heats of reaction shown in the table below can be obtained experimentally or looked up in tables of enthalpy data. which two of these heats of reaction would be the easiest and safest to measure in the laboratory, and which two are better obtained through reference sources? why? hint: consider whether a reaction takes place in aqueous solution or instead involves noxious gases.
this is the equation for a: δh25° = ( δh25° nh3 + δh25° hcl) - ( δh25° nh4cl)
and attached is the table

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
The heat of reaction for the process described in (a) can be determined by applying hess's law. the...
Questions

Mathematics, 01.10.2021 23:50




Spanish, 01.10.2021 23:50

History, 01.10.2021 23:50



English, 02.10.2021 01:00


Mathematics, 02.10.2021 01:00

Biology, 02.10.2021 01:00

Mathematics, 02.10.2021 01:00

English, 02.10.2021 01:00


Mathematics, 02.10.2021 01:00






