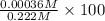Chemistry, 29.08.2019 18:00 lhmsokol56
Calculate the percent ionization of nitrous acid in a solution that is 0.222 m in nitrous acid (hno3) and 0.278 m in potassium nitrite (kno2). the acid dissociation constant of nitrous acid is 4.50 x 10-4 a) 55.6 b) 0.162 c) 15.5 d) 2.78 * 10-3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Calculate the percent ionization of nitrous acid in a solution that is 0.222 m in nitrous acid (hno3...
Questions

Mathematics, 21.10.2020 17:01

English, 21.10.2020 17:01

Arts, 21.10.2020 17:01



History, 21.10.2020 17:01



Computers and Technology, 21.10.2020 17:01


Mathematics, 21.10.2020 17:01





Social Studies, 21.10.2020 17:01

Chemistry, 21.10.2020 17:01

Mathematics, 21.10.2020 17:01

Physics, 21.10.2020 17:01

Social Studies, 21.10.2020 17:01

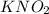 is 0.278 M and it is completely ionized into
is 0.278 M and it is completely ionized into 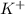 and
and 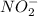 .
.![[KNO_{2}]](/tpl/images/0209/3896/fb191.png) =
= ![[NO_{2}]](/tpl/images/0209/3896/53e25.png) = 0.278 M
= 0.278 M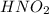 is 0.222 M.
is 0.222 M.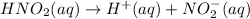
![K_{a} = \frac{[H^{+}][NO^{-}_{2}]}{[HNO_{2}]}](/tpl/images/0209/3896/218b7.png)
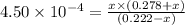
![[H^{+}]](/tpl/images/0209/3896/85507.png) is 0.00036 M. Therefore, percentage ionization of
is 0.00036 M. Therefore, percentage ionization of ![\frac{[H^{+}]}{[HNO_{2}]} \times 100](/tpl/images/0209/3896/26d17.png)