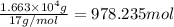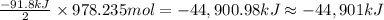Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 15:30
Select the correct answer.the gas in a sealed container has an absolute pressure of 125.4 kilopascals. if the air around the container is at a pressure of 99.8 kilopascals, what is thegauge pressure inside the container?
Answers: 3

Chemistry, 23.06.2019 16:30
There is a set up transformer that doubles the voltage. if the primary coil has a voltage of 10 v
Answers: 2
You know the right answer?
The production of ammonia (nh3) under standard conditions at 25°c is represented by the following th...
Questions

History, 24.08.2020 19:01



Mathematics, 24.08.2020 19:01



English, 24.08.2020 19:01

History, 24.08.2020 19:01

Chemistry, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01


History, 24.08.2020 19:01

Health, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01


Mathematics, 24.08.2020 19:01

History, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01

Mathematics, 24.08.2020 19:01

Medicine, 24.08.2020 19:01

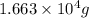 grams of ammonia is produced.
grams of ammonia is produced.