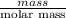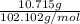Chemistry, 29.08.2019 19:10 sarmientojose267
Four beakers containing potassium nitrate dissolved in water are allowed to evaporate to dryness. beakers 1 through 4 contain 2.3, 1.91, 5.985, and 0.52 g of dry potassium nitrate respectively. how many moles of potassium nitrate were recovered after the water evaporated?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Four beakers containing potassium nitrate dissolved in water are allowed to evaporate to dryness. be...
Questions

Mathematics, 22.06.2019 09:40

Mathematics, 22.06.2019 09:40







Mathematics, 22.06.2019 09:40

Social Studies, 22.06.2019 09:40








Chemistry, 22.06.2019 09:40


History, 22.06.2019 09:40

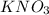 = molar mass of K + molar mass of N + 3 × molar mass of O
= molar mass of K + molar mass of N + 3 × molar mass of O