
Chemistry, 29.08.2019 19:20 ravenhudsonmail46
2agno3(aq) + nas(aq) + 40200 how many milliliters of 0.225 m nh, solution will exactly react with 30.0 ml of a 0.190 m h2so4 solution? 2nh3(aq) + h2so4(aq) → (nh4)2so4(aq) - 2+1

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
2agno3(aq) + nas(aq) + 40200 how many milliliters of 0.225 m nh, solution will exactly react with 30...
Questions

Mathematics, 06.11.2020 06:00

Chemistry, 06.11.2020 06:00



Social Studies, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00

Medicine, 06.11.2020 06:00

English, 06.11.2020 06:00


History, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00


Mathematics, 06.11.2020 06:00

History, 06.11.2020 06:00


Biology, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00


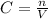
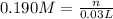 ∴ n = 5.7 x 10⁻³ mol
∴ n = 5.7 x 10⁻³ mol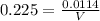 ∴ V = 50.67 mL
∴ V = 50.67 mL

