
Chemistry, 29.08.2019 20:20 jessnolonger
Carbon, hydrogen and ethane each burn exothermically in an excess of air. ahⓡ =-393.7 kj mol. c(s) + o2(g) → co2(g) h2(g) + % o2(g) → h20(1) czha(g) + 302() → 2co2(g) + 2h2o(1) ah®=-285.9 kj mol ah =-1411.0 kj moll. use the data to calculate the standard enthalpy change of formation, ah in kj mol'', of ethene at 298 k 2c(s) + 2h2(g) → c2h4(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Carbon, hydrogen and ethane each burn exothermically in an excess of air. ahⓡ =-393.7 kj mol. c(s) +...
Questions

History, 03.07.2019 20:50

History, 03.07.2019 20:50

Social Studies, 03.07.2019 20:50

Business, 03.07.2019 20:50

Business, 03.07.2019 21:00


Mathematics, 03.07.2019 21:00

Social Studies, 03.07.2019 21:00


English, 03.07.2019 21:00


Mathematics, 03.07.2019 21:00

Mathematics, 03.07.2019 21:00


Mathematics, 03.07.2019 21:00

Social Studies, 03.07.2019 21:00



Business, 03.07.2019 21:00

Business, 03.07.2019 21:00

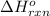 for the reaction is 51.8 kJ.
for the reaction is 51.8 kJ.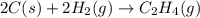
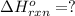
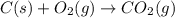
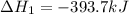 ( × 2)
( × 2)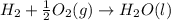
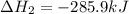 ( × 2)
( × 2)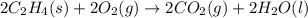

![\Delta H^o_{rxn}=[2\times \Delta H_1]+[2\times \Delta H_2]+[1\times (-\Delta H_3)]](/tpl/images/0209/7515/e45ac.png)
![\Delta H^o_{rxn}=[(2\times (-393.7))+(2\times (-285.9))+(1\times -(-1411))]=51.8kJ](/tpl/images/0209/7515/5c6c6.png)


