
Chemistry, 30.08.2019 00:30 mixcolin0002
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc sulfate. explain why there will be no reaction when zinc metal and aqueous copper sulfate solution are combined. identify the anode and the cathode, assuming a voltaic cell is constructed. note: be careful in the calculation of the standard cell potential ( eo cathode - eo anode). do not change the sign of the given reduction potential. the sign is already taken care of using the formula for calculating the standard cell potential.
standard reduction potential: cu2+(aq) + 2e- > cu(s) eo = -0.34 v
zn2+(aq) + 2e- > zn(s) eo = -0.76 v
standard cell potential = eo cathode - eo anode
although , combination of the reactants do not result in voltaic cells, it can be predicted if the reaction is spontaneous, based on the standard reduction potential.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3


Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc s...
Questions

Mathematics, 16.10.2020 08:01








Advanced Placement (AP), 16.10.2020 08:01


Geography, 16.10.2020 08:01

Social Studies, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

English, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

History, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01


Geography, 16.10.2020 08:01

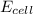 = +ve, reaction is spontaneous
= +ve, reaction is spontaneous
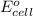 = standard electrode potential =
= standard electrode potential =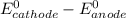
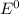 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Cu^{2+}/Cu]}= +0.34V](/tpl/images/0210/3859/55543.png)
![E^0_{[Zn^{2+}/Zn]}= -0.76V](/tpl/images/0210/3859/6c8c3.png)
![E^0=E^0_{[Zn^{2+}/Zn]}- E^0_{[Cu^{2+}/Cu]}](/tpl/images/0210/3859/f1898.png)


![E^0=E^0_{[Cu^{2+}/Cu]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0210/3859/cb03b.png)



