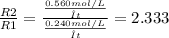Chemistry, 30.08.2019 02:30 cupcake3103670
Astudent sets up two reactions. reaction 1 uses 0.240 mol/l of reactant, and reaction 2 uses 0.560 mol/l of reactant. how many times faster is reaction 2 compared to reaction 1?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
Astudent sets up two reactions. reaction 1 uses 0.240 mol/l of reactant, and reaction 2 uses 0.560 m...
Questions


Health, 20.09.2020 02:01


Social Studies, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01

History, 20.09.2020 02:01

English, 20.09.2020 02:01

French, 20.09.2020 02:01



Mathematics, 20.09.2020 02:01


English, 20.09.2020 02:01

Spanish, 20.09.2020 02:01

English, 20.09.2020 02:01

History, 20.09.2020 02:01



![rate = \frac{[reactant]}{Δtime}](/tpl/images/0210/6835/eeab7.png)
![R1 = \frac{[0.240 mol/L]}{Δt} \\R2 = \frac{[0.560 mol/L}{Δt}](/tpl/images/0210/6835/942fb.png)