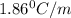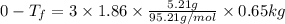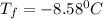Chemistry, 30.08.2019 03:10 desdes1499
If 95.21 g of mgcl2, a strong electrolyte is added to 650 g of water, how much would the freezing point of water be lowered? what would be freezing point of this solution? the kf for water is 1.86 degrees c/m.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
If 95.21 g of mgcl2, a strong electrolyte is added to 650 g of water, how much would the freezing po...
Questions






Chemistry, 25.06.2019 02:40

History, 25.06.2019 02:40






Mathematics, 25.06.2019 02:50




English, 25.06.2019 02:50

Mathematics, 25.06.2019 02:50

Mathematics, 25.06.2019 02:50

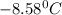
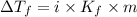
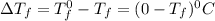 = Depression in freezing point
= Depression in freezing point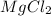 as it dissociates to give three ions.
as it dissociates to give three ions.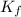 = freezing point constant =
= freezing point constant =