
Chemistry, 30.08.2019 04:10 malasyamcclendon
3cu(s) + 8 hno3(aq) + 3 cu(no3)2(aq) + 2no(9) + 4h20() c) if 5.58 g of copper(ii) nitrate, cu(no3)2, is eventually obtained, how many moles of nitric acid, hno3, were used in the experiment?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
3cu(s) + 8 hno3(aq) + 3 cu(no3)2(aq) + 2no(9) + 4h20() c) if 5.58 g of copper(ii) nitrate, cu(no3)2,...
Questions

Mathematics, 21.03.2021 05:40

Computers and Technology, 21.03.2021 05:40

Mathematics, 21.03.2021 05:50


Mathematics, 21.03.2021 05:50

Mathematics, 21.03.2021 05:50

Social Studies, 21.03.2021 05:50

Mathematics, 21.03.2021 05:50




Arts, 21.03.2021 05:50

Mathematics, 21.03.2021 05:50


Business, 21.03.2021 05:50


Mathematics, 21.03.2021 05:50

Mathematics, 21.03.2021 05:50


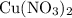 :
: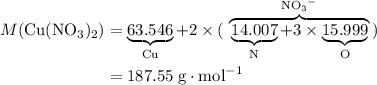 .
.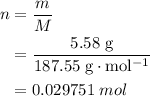 .
.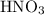 and that of
and that of 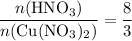 .
.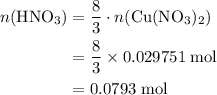 .
.


