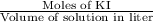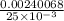Chemistry, 30.08.2019 05:30 xxaurorabluexx
The concentration of a potassium iodide solution can be determined by first adding excess silver nitrate: i'(aq) + ag+ (aq) → agl(s) the excess silver ion remaining in solution is then determined by reaction with a potassium thiocyanate (kscn) solution of known concentration: ag (aq) + scn(aq) → agscn(s) in an experiment, 50.00 ml of 0.0565 m agno3 was added to 25.00 ml of a potassium iodide solution. it then took 8.32 ml of 0.0510 m kscn solution to precipitate the unreacted silver ions. what is the concentration of the original ki solution?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1


Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

You know the right answer?
The concentration of a potassium iodide solution can be determined by first adding excess silver nit...
Questions


Mathematics, 16.06.2021 18:20



Social Studies, 16.06.2021 18:20


Mathematics, 16.06.2021 18:20


English, 16.06.2021 18:20


History, 16.06.2021 18:20



History, 16.06.2021 18:20


Social Studies, 16.06.2021 18:20

Mathematics, 16.06.2021 18:20