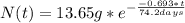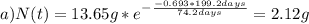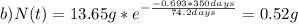Chemistry, 30.08.2019 16:10 hlgerardip4wbhx
The half-life for the radioactive decay of iridium-192 is 74.2 days. calculate the amount in grams of ir-192 that will be left from a 13.65g sample after a) 199.2 days b) 350 days

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
The half-life for the radioactive decay of iridium-192 is 74.2 days. calculate the amount in grams o...
Questions


Mathematics, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40


Mathematics, 11.11.2020 23:40


English, 11.11.2020 23:40

SAT, 11.11.2020 23:40

English, 11.11.2020 23:40

English, 11.11.2020 23:40


Mathematics, 11.11.2020 23:40

Social Studies, 11.11.2020 23:40


Mathematics, 11.11.2020 23:40

Physics, 11.11.2020 23:40


Business, 11.11.2020 23:40

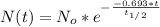
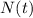 is the amount given a certain t time, and
is the amount given a certain t time, and 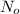 is the initial amount.
is the initial amount. 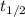 is the half life.
is the half life.