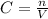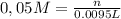Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an indicator solution and titrates with sodium hydroxide. he finds the equivalence point occurs when he's titrated 9.5 ml of 0.05 m sodium hydroxide. approximately how much chlorogenic acid is in the sample? 475 umol ® 480 umol © 500 umol 510 umol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an in...
Questions




Spanish, 17.12.2019 17:31

Mathematics, 17.12.2019 17:31

Biology, 17.12.2019 17:31


Mathematics, 17.12.2019 17:31


Mathematics, 17.12.2019 17:31




Arts, 17.12.2019 17:31



Mathematics, 17.12.2019 17:31

Biology, 17.12.2019 17:31