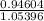Chemistry, 30.08.2019 17:20 brisamauro27
Calculate the change in ph when 71.0 ml of a 0.760 m solution of naoh is added to 1.00 l of a solution that is 1.0o m in sodium acetate and 1.00 m in acetic acid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
Calculate the change in ph when 71.0 ml of a 0.760 m solution of naoh is added to 1.00 l of a soluti...
Questions

History, 07.10.2019 16:00

Mathematics, 07.10.2019 16:00





Social Studies, 07.10.2019 16:00

English, 07.10.2019 16:00

Mathematics, 07.10.2019 16:00


History, 07.10.2019 16:00

Social Studies, 07.10.2019 16:00

Mathematics, 07.10.2019 16:00

History, 07.10.2019 16:00

Mathematics, 07.10.2019 16:00

Biology, 07.10.2019 16:00


Health, 07.10.2019 16:00


Mathematics, 07.10.2019 16:00

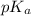 value of acetic acid is 4.74. And, relation between pH and
value of acetic acid is 4.74. And, relation between pH and ![\frac{[CH_{3}COOH]}{[CH_{3}COONa]}](/tpl/images/0212/4640/b577a.png)
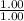
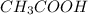 = moles of
= moles of 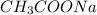
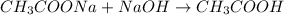
![\frac{[CH_{3}COONa]}{[CH_{3}COOH]}](/tpl/images/0212/4640/ccc54.png)