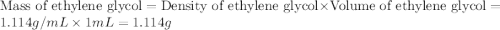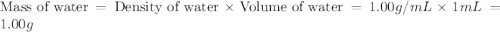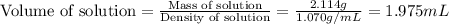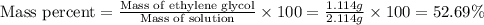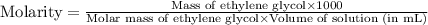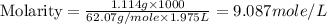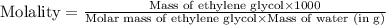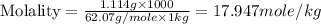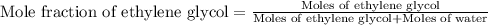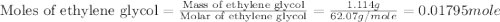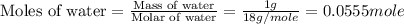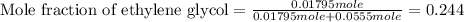An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml; m = 62.07 g/mol) and water (d = 1.00 g/ml) at 20°c. the density of the mixture is 1.070 g/ml. express the concentration of ethylene glycol as (a) volume percent 50 % v/v (b) mass percent 52.7 % w/w (c) molarity m (d) molality m (e) mole fraction

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 17:30
Hydrogen-2 is also known as deuterium as well as hydrogen-3 is known as tritium hydrogen-1 is our common hydrogen isotope a sample hydrogen gas has 99% hydrogen -1 ,0.8% deuterium , and 0.2% tritium what is the average atomic mass of this mixture of isotope to the thousands place
Answers: 1

Chemistry, 23.06.2019 21:00
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and second order in c. by what factor does the reaction rate change if the concentration of a is doubled? 1 by what factor does the reaction rate change if the concentration of b is doubled? 1.4 by what factor does the reaction rate change if the concentration of c is doubled? 4
Answers: 1
You know the right answer?
An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml;...
Questions


Mathematics, 20.03.2020 09:47







Computers and Technology, 20.03.2020 09:47




Mathematics, 20.03.2020 09:48




Physics, 20.03.2020 09:48