
Chemistry, 30.08.2019 22:30 ayaanwaseem
Calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. it takes 4.2 joules to change 1.0 grm (1.0 ml) of water 1.0 celcius. is it endotherrmic or exothermic? volume of vinegar 25 ml, initial temp of vinegar 17 celcius and final temp is 14 celcius.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Pauling and lewis questioned the extreme definitions of bonds. they wondered if bonds might be described somewhere in between the two extremes (covalent and ionic). on the basis of experimental data,pauling confirmed that bonds could be ionic, covalent, and for those, in between, exhibit a degree of ionic character. he theorized that the major factor was how strongly the atoms in the bond attracted the electrons. pauling called this factor - the tendency of an atom to attract electrons in a bond.
Answers: 2

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2
You know the right answer?
Calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has th...
Questions

English, 05.01.2020 02:31

Computers and Technology, 05.01.2020 02:31

Biology, 05.01.2020 03:31

Mathematics, 05.01.2020 03:31

History, 05.01.2020 03:31

Mathematics, 05.01.2020 03:31

Biology, 05.01.2020 03:31


Health, 05.01.2020 03:31


English, 05.01.2020 03:31




History, 05.01.2020 03:31





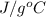
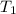 =
= 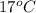 , and
, and 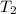 =
= 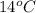
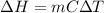
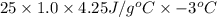 (as density =
(as density = 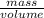 )
)


