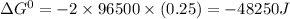11. (12pts) for the redox reaction given below, ni(s) + 2 h(aq) + ni2+(aq) + h2(e) (show your work in detail) a) write oxidation and reduction half cell reactions b) write the electrochemical cell notation c) predict whether this reaction will be spontaneous. explain. d) calculategº 6 page

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
11. (12pts) for the redox reaction given below, ni(s) + 2 h(aq) + ni2+(aq) + h2(e) (show your work i...
Questions

Physics, 22.09.2019 16:30


Mathematics, 22.09.2019 16:30

History, 22.09.2019 16:30

Mathematics, 22.09.2019 16:30

Computers and Technology, 22.09.2019 16:30

Arts, 22.09.2019 16:30


Mathematics, 22.09.2019 16:30

Biology, 22.09.2019 16:30

Geography, 22.09.2019 16:30


Mathematics, 22.09.2019 16:30

Chemistry, 22.09.2019 16:30


Mathematics, 22.09.2019 16:30

Mathematics, 22.09.2019 16:30



History, 22.09.2019 16:30

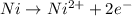
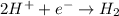
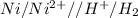
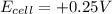 , the reaction is spontaneous.
, the reaction is spontaneous.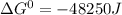
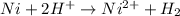
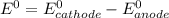
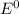 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Ni^{2+}/Ni]}= -0.25V](/tpl/images/0213/4556/864ce.png)
![E^0_{[H^{+}/H_2]}=+0.0V](/tpl/images/0213/4556/826b0.png)
![E^0=E^0_{[H^{+}/H_2]}- E^0_{[Ni^{2+}/Ni]}](/tpl/images/0213/4556/3d59c.png)
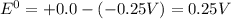
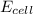 = +ve, reaction is spontaneous
= +ve, reaction is spontaneous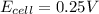 , the reaction is spontaneous.
, the reaction is spontaneous.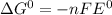
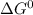 = standard gibbs free energy
= standard gibbs free energy