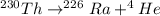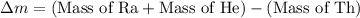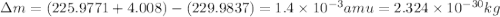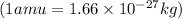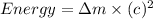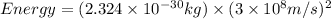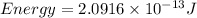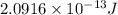Chemistry, 31.08.2019 00:20 angelinaranee15
How much energy is released by the decay of 3 grams of 20th in the following reaction 230 th - 226ra + 'he (230 th = 229.9837 g/mol, 226ra - 225.9771 g/mol, "he = 4.008 g/mol) (10 pts.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
How much energy is released by the decay of 3 grams of 20th in the following reaction 230 th - 226ra...
Questions

Physics, 12.02.2020 00:30



Chemistry, 12.02.2020 00:30



Mathematics, 12.02.2020 00:30





Mathematics, 12.02.2020 00:30

Chemistry, 12.02.2020 00:30

Chemistry, 12.02.2020 00:30






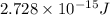
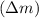 .
.