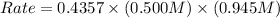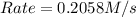Chemistry, 31.08.2019 01:00 erikamaldonado661
For the following reaction: 2a +bő20 the rate law is determined to be: rate = 0.4357 [a][b] what will be the initial rate (in m/s) if initial concentrations are: [a] = 0.500 m, [b] = 0.945 [m]

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
For the following reaction: 2a +bő20 the rate law is determined to be: rate = 0.4357 [a][b] what w...
Questions

English, 07.11.2020 19:50

English, 07.11.2020 19:50





Social Studies, 07.11.2020 19:50

Mathematics, 07.11.2020 19:50

Biology, 07.11.2020 19:50

World Languages, 07.11.2020 19:50

Mathematics, 07.11.2020 19:50


Mathematics, 07.11.2020 19:50


Biology, 07.11.2020 19:50

Social Studies, 07.11.2020 19:50

English, 07.11.2020 19:50

Mathematics, 07.11.2020 19:50


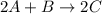
![Rate=0.4357[A][B]](/tpl/images/0213/5518/5bee5.png)