
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 124.0°c. (cwater 118 jig'c, gee 2.03 jig c, g team jig c, molar heat of fusion of ice 6.01 * 10 j/mol; molar heat of vaporization of liquid water 4.07 * 10*j/mol 202 short answer toolbar navigation b i v s e 1 e a a this question will be sent to your instructor for grading

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 1...
Questions


Business, 21.04.2020 01:58

Mathematics, 21.04.2020 01:58


Chemistry, 21.04.2020 01:58



Mathematics, 21.04.2020 01:58


Mathematics, 21.04.2020 01:59






Chemistry, 21.04.2020 01:59


History, 21.04.2020 01:59

Chemistry, 21.04.2020 01:59

Physics, 21.04.2020 01:59


![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0213/6323/e4ef0.png)
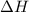 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?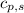 = specific heat of solid water =
= specific heat of solid water = 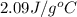
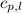 = specific heat of liquid water =
= specific heat of liquid water = 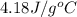
 = specific heat of liquid water =
= specific heat of liquid water = 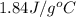
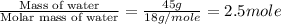
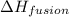 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole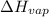 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[45g\times 4.18J/gK\times (0-(-15.5))^oC]+2.5mole\times 6010J/mole+[45g\times 2.09J/gK\times (100-0)^oC]+2.5mole\times 40670J/mole+[45g\times 1.84J/gK\times (124-100)^oC]](/tpl/images/0213/6323/555ef.png)
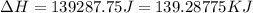 (1 KJ = 1000 J)
(1 KJ = 1000 J)

