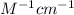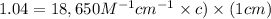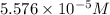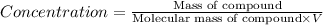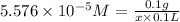Chemistry, 31.08.2019 01:30 alexisscalera7
Amolecule is known to have a molar absorptivity of 18,650 at a certain wavelength. a spectrometer is tuned to this wavelength and used to measure the absorbance of a solution of this substance in a b = 1 cm cuvette. the indicated absorbance of the sample was 1.04. the tested solution used in the experiment was prepared by dissolving 100 mg of the substance into a 100 ml volumetric flask. what is the molecular weight of the substance / molecule tested?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
Amolecule is known to have a molar absorptivity of 18,650 at a certain wavelength. a spectrometer is...
Questions



English, 04.06.2020 17:58


History, 04.06.2020 17:58

Mathematics, 04.06.2020 17:58

Business, 04.06.2020 17:58

Mathematics, 04.06.2020 17:58


Mathematics, 04.06.2020 17:58

Mathematics, 04.06.2020 17:58



Mathematics, 04.06.2020 17:59



English, 04.06.2020 17:59


Biology, 04.06.2020 17:59

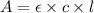
 = molar absorptivity of this solution = 18,650
= molar absorptivity of this solution = 18,650