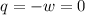Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calculate w, q and deltau for each of the following situations: (i) a reversible expansion of the sample. (ii) an irreversible expansion of the sample against a constant external pressure equal to the final pressure of the gas. (iii) a free expansion (against zero external pressure i. e. in a vacuum) of the sample.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calcu...
Questions

Mathematics, 30.10.2020 22:30

Mathematics, 30.10.2020 22:30

Social Studies, 30.10.2020 22:30



Mathematics, 30.10.2020 22:30



Mathematics, 30.10.2020 22:30

English, 30.10.2020 22:30

Mathematics, 30.10.2020 22:30


Chemistry, 30.10.2020 22:30

Geography, 30.10.2020 22:30

History, 30.10.2020 22:30

Mathematics, 30.10.2020 22:30

English, 30.10.2020 22:30



Mathematics, 30.10.2020 22:30

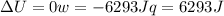
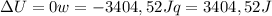
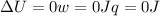
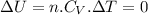 since
since 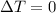
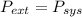 so
so 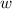 can be calculated by
can be calculated by 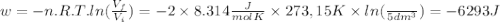
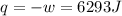
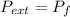
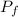 by the law of ideal gases
by the law of ideal gases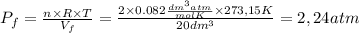
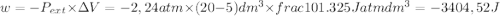
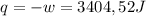
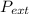 so
so 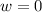 (there's no work at vaccum) and
(there's no work at vaccum) and