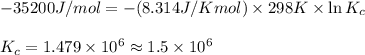What is the equilibrium constant for the reaction:
so2 (g) + no2 (g) â so3 (g) + no (g)
...

Chemistry, 31.08.2019 02:30 cynayapartlow88
What is the equilibrium constant for the reaction:
so2 (g) + no2 (g) â so3 (g) + no (g)
at 298 k? use the following data: r=8.314 j/(k. mol)
substance so2 (g) so3 (g) no2 (g) no (g)
îgo (kj/mol) -300.2 -371 51 86.6
a) 6.8 . 10-7
b) 1.5 . 106
c) 1.014
d) 0.986
e) -35.2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
Questions

Computers and Technology, 30.09.2019 18:30

History, 30.09.2019 18:30











Mathematics, 30.09.2019 18:30

Physics, 30.09.2019 18:30






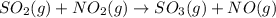
![\Delta G^o_{rxn}=\sum [\Delta G^o_f(product)]-\sum [\Delta G^o_f(reactant)]](/tpl/images/0213/7584/a3f80.png)
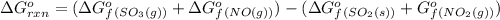
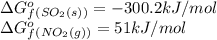
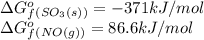
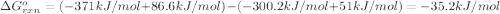
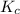 (at 298 K) for given value of Gibbs free energy, we use the relation:
(at 298 K) for given value of Gibbs free energy, we use the relation: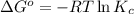
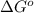 = Gibbs free energy = -35.2 kJ/mol = -35200 J/mol (Conversion factor: 1kJ = 1000J)
= Gibbs free energy = -35.2 kJ/mol = -35200 J/mol (Conversion factor: 1kJ = 1000J)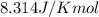
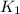 = equilibrium constant at 298 k;
= equilibrium constant at 298 k;