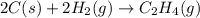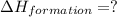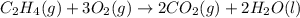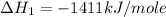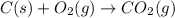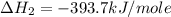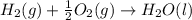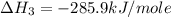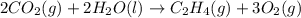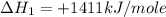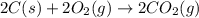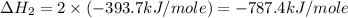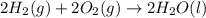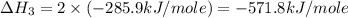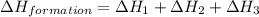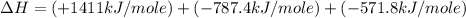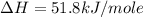Chemistry, 31.08.2019 04:10 isabella4141
How to draw hess' cycle for this question ?
carbon, hydrogen and ethane each burn exothermically in an excess of air. c(s) + o2(g) → co2(g) ahⓡ =-393.7 kj mol: 1. h2(g) + % o2(g) → h2o(1) ah® --285.9 kj mol. c2h4(g) + 302(g) → 2co2(g) + 2h2o(1) ah® --1411.0 kj mol? . use the data to calculate the standard enthalpy change of formation, ah® in kj mol? , of ethene at 298 k 2c(s) + 2h2(g) → c2h4(8)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
How to draw hess' cycle for this question ?
carbon, hydrogen and ethane each burn exothermica...
carbon, hydrogen and ethane each burn exothermica...
Questions

Mathematics, 11.03.2020 03:00

Mathematics, 11.03.2020 03:00



Mathematics, 11.03.2020 03:01

Mathematics, 11.03.2020 03:01

Mathematics, 11.03.2020 03:01



Geography, 11.03.2020 03:01










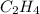 will be,
will be,