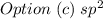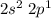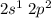Chemistry, 31.08.2019 04:30 edgarlimon2000
Which of the following hybrid atomic orbitals would you consider most useful for approximating a molecular orbital scheme around the boron atom in bf3?
select one:
a. sp3
b. 2s and 2p
c. sp2
d. sp

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
Which of the following hybrid atomic orbitals would you consider most useful for approximating a mol...
Questions

Mathematics, 02.10.2019 07:30

English, 02.10.2019 07:30

Biology, 02.10.2019 07:30

Mathematics, 02.10.2019 07:30



History, 02.10.2019 07:30


Mathematics, 02.10.2019 07:30


Mathematics, 02.10.2019 07:30

Health, 02.10.2019 07:30

Geography, 02.10.2019 07:30

Chemistry, 02.10.2019 07:30

Biology, 02.10.2019 07:30



History, 02.10.2019 07:30


English, 02.10.2019 07:30