The following data was measured for the reaction above at a constant temperature.
experiment i...

Chemistry, 31.08.2019 05:30 4300224102
The following data was measured for the reaction above at a constant temperature.
experiment initial [a] mol l‐1 initial [b] mol l‐1 initial rate of formation of c (mol l‐1 s‐1)
1 0.10 0.10 1.12 x 10‐3
2 0.10 0.20 2.24 x 10‐3
3 0.20 0.10 4.48 x 10‐3
what is the order of the reaction with respect to a?
what is the order of the reaction with respect to b?
what is the rate law for this reaction?
calculate the rate constant.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Questions

Mathematics, 06.01.2020 00:31


Social Studies, 06.01.2020 00:31

Geography, 06.01.2020 00:31





Mathematics, 06.01.2020 00:31



Mathematics, 06.01.2020 00:31


Health, 06.01.2020 00:31


Social Studies, 06.01.2020 00:31

Mathematics, 06.01.2020 00:31

Mathematics, 06.01.2020 00:31

Geography, 06.01.2020 00:31

History, 06.01.2020 00:31

![V=k[A]^{2} [B]](/tpl/images/0214/1245/29401.png)
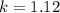
![\frac{[A]_{3}}{[A]_1{}} =2](/tpl/images/0214/1245/57061.png)
![\frac{[V]_{3}}{[V]_1{}} =4](/tpl/images/0214/1245/908c3.png)
![[A]^{2}](/tpl/images/0214/1245/66fd2.png)
![\frac{[B]_{2}}{[B]_1{}} =2](/tpl/images/0214/1245/5cc2d.png)
![\frac{[V]_{2}}{[V]_1{}} =2](/tpl/images/0214/1245/0fa6f.png)
![[B]}](/tpl/images/0214/1245/fe23b.png)
![V=k[A]^{2}[B]](/tpl/images/0214/1245/202fc.png)
![k=\frac{V}{[A]^{2}[B]}](/tpl/images/0214/1245/4243e.png)


