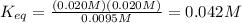Consider the reaction below.
at 500 k, the reaction is at equilibrium with the following conce...

Chemistry, 01.09.2019 17:20 vanleervanleer
Consider the reaction below.
at 500 k, the reaction is at equilibrium with the following concentrations.
[pci5]= 0.0095 m
[pci3] = 0.020
[ci2] = 0.020 m
what is the equilibrium constant for the given reaction?
0.042
0.42
2.4
24
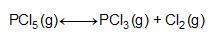

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Questions


Spanish, 25.03.2021 18:30

English, 25.03.2021 18:30

English, 25.03.2021 18:30


Mathematics, 25.03.2021 18:30

Business, 25.03.2021 18:30

Mathematics, 25.03.2021 18:30







Mathematics, 25.03.2021 18:30




Mathematics, 25.03.2021 18:30

![K_{eq}=\frac{[PCl_3]^1[Cl_2]^1}{[PCl_5]^1}](/tpl/images/0218/2590/d25fc.png)